Kerry announces licensing agreement for rice-derived postbiotic
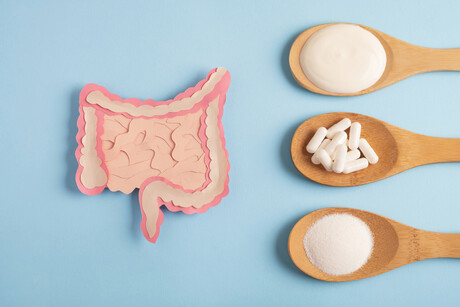
Kerry has announced a commercial agreement with Japan-based Kameda Seika to market and distribute the company’s rice-derived postbiotic across a range of applications in Europe and the Americas.
Kameda Seika is a global rice cracker company that has researched rice-based lactobacillus for over 25 years. Its postbiotic Lactobacillus K-1 (Lactobacillus casei subsp, 327) is claimed to improve both digestive and skin health and can be used in food, beverages and supplements.
Postbiotics are the metabolic by-products of fermentation. They offer similar functionality to probiotics and are backed by growing clinical evidence. Manufacturers may use them as a heat- and acid-stable health-enhancing ingredient.
Kerry will leverage Kameda’s technology portfolio, application expertise and commercial footprint to drive the commercialisation of the technology to reach users outside of Japan.
Lactobacillus K-1 was tested in a randomised, double-blind, placebo-controlled study which found that it may improve digestive health. Results were further supported by additional scientific data which found that it may improve gut and skin health.
Chemical food additive BHA under review in the US
The FDA identified butylated hydroxyanisole (BHA) as a top priority for review as part of its...
Infant formula recalled due to potential toxin contamination
Due to the potential presence of the toxin cereulide, there has been a precautionary recall of...
Call for comment on a new source of 2′-FL in infant formula products
FSANZ is calling for comments on an application to permit 2′-fucosyllactose produced from a...











