Designer meat — one step closer

Lab-grown or cultured meat is touted as a means of providing a greener, more sustainable alternative to meat production. However, more work is required to develop large-scale production and improvements in the feel and taste of the product.
Inspired by the process of creating fairy floss, researchers at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have now grown rabbit and cow muscles cells on edible gelatin fibres produced by 'immersion rotary jet spinning' to mimic the texture and consistency of meat. The research has been published in Nature Science of Food.
Animal meat consists mostly of skeletal muscle (and fat tissue) which grows in long, thin fibres — as can be seen in the grain of a steak or when shredding pork or chicken. Reproducing these fibres is one of the biggest challenges in bioengineering meat.
"Muscle cells are adherent cell types, meaning they need something to hold onto as they grow," said Luke Macqueen, first author of the study and postdoctoral fellow at SEAS and the Wyss Institute for Bioinspired Engineering. "To grow muscle tissues that resembled meat, we needed to find a 'scaffold' material that was edible and allowed muscle cells to attach and grow in 3D. It was important to find an efficient way to produce large amounts of these scaffolds to justify their potential use in food production."
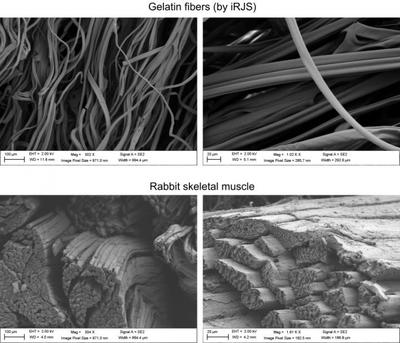
To overcome these challenges, the researchers used a technique developed by Parker and his Disease Biophysics Group known as immersion Rotary Jet-Spinning (iRJS), which uses centrifugal force to spin long nanofibres of specific shapes and sizes. The team spun food-safe gelatin fibres to form the base for growing cells. The fibres mimic natural muscle tissue's extracellular matrix — the glue that holds the tissue together and contributes to its texture.

The team seeded the fibres with rabbit and cow muscle cells, which anchored to the gelatin and grow in long, thin structures, similar to real meat. The researchers used mechanical testing to compare the texture of their lab-grown meat to real rabbit, bacon, beef tenderloin, prosciutto and other meat products.
"When we analysed the microstructure and texture, we found that, although the cultured and natural products had comparable texture, natural meat contained more muscle fibres, meaning they were more mature," Macqueen said. "Muscle and fat cell maturation in vitro are still a really big challenge that will take a combination of advanced stem cell sources, serum-free culture media formulations, edible scaffolds such as ours, as well as advances in bioreactor culture methods to overcome."
Still, this research shows that full lab-grown meat is possible.
"Eventually, we think it may be possible to design meats with defined textures, tastes, and nutritional profiles — a bit like brewing," Macqueen said.
Smart optical sensor detects ripeness for a quality drop
Queen Mary University of London researchers are developing a handheld, AI-powered grape ripeness...
Lighting up wine spoilage in real time with novel biosensor
A biosensor that glows when acetic acid is detected has been built by researchers to help...
Novel food processing technologies retain more nutrients in food
The potential of a range of modern techniques to improve the nutritional profiles of grain-,...











